
T-SPOT.TB 혈액 검사의 장점
T-SPOT.TB 검사는 세포 수와 배양 조건 모두에 대해 유일하게 전 세계적으로 이용 가능한 표준화된 IGRA입니다. 이 검사는 세포 수를 표준화하고 검사 결과에 부정적인 영향을 미칠 수 있는 혈청 인자를 제거해 결핵 감염에 대한 가장 민감하고 특이적인 검사입니다.2,3 면역 억제를 포함한 모든 환자 그룹에서 결핵 감염을 빠르고 안정적으로 진단하고 치료할 수 있습니다.4
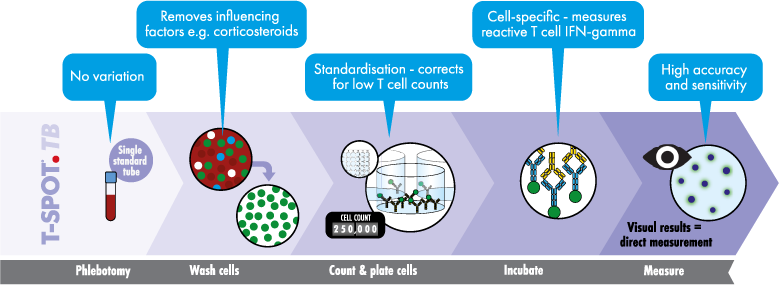
T-SPOT.TB 검사의 장점

-
Accurate across patient populations4
- 면역 억제
- BCG 예방 접종
-
일관된 결과6,7
- 민감도: 98.8 %
- 특이도:> 99.1
-
승인된 borderline 구역으로 인해 결과 컷오프에 대해 검사 정확도가 높음 – 부적절한 치료 방지에 도움을 줌2,5
T-SPOT.TB 검사 자동화2,3
CE 마크가 있는 T-Cell Select
T-SPOT.TB 검사의 검체 안정성 향상!

T-Cell Xtend 시약은 T-SPOT.TB 검사를 시행하기 직전에 검사실의 혈액 검체에 추가되는 항체 복합체로, 검사의 정확성에 영향을 주지 않고 정맥 채혈 후 최대 32 시간까지 혈액 검체를 처리할 수 있습니다8

이제 CE 마크가 있는 T-Cell Select 시약 키트를 사용하여 T-SPOT.TB 검사를 자동화할 수 있습니다. T-Cell Select 시약 키트를 사용하면 T-SPOT.TB 검사용 검체 채혈후 최대 54 시간까지 처리할 수 있습니다. 정맥 채혈 후 검사실에서 검체 처리까지 2일이 넘는 시간입니다.
Learn more about the
T-SPOT.TB test
GET STARTED
- Global tuberculosis report 2020. Geneva: World Health Organization; 2020. CC BY-NC-SA 3.0 IGO
- Oxford Immunotec. T-SPOT.TB Package Insert PI-TB-IVD-UK-v3. Abingdon, UK. 2016.
- Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American ThoracicSociety/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Infect Dis. Published online December 8, 2016:ciw694. doi:10.1093/cid/ciw694
- Wong SH, Gao Q, Tsoi KKF, et al. Effect of immunosuppressive therapy on interferon γ release assay for latent tuberculosis screening in patients with autoimmune diseases: a systematic review and meta-analysis. Thorax. 2016;71:64–72.
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, IGRA Expert Committee, Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection – United States, 2010. MMWR Recomm Rep. 2010; 59(RR-5:1-25.
- WHO End TB Strategy. World Health Organization. Published September 8, 2015. Accessed November 12, 2020.
- Wrighton-Smith P, Sneed L, Humphrey F, Tao X, Bernacki E. Screening health care workers with interferon-γ release assay versus tuberculin skin test: Impact on costs and adherence to testing (the SWITCH study). J Occupational & Environmental Med. 2012;54(7):806-815
- Oxford Immunotec. T-Cell Xtend Package Insert. PI-TT.610-UK-V6. Abingdon, UK. 2017.
- Tuberculosis (TB). Centers for Disease Control and Prevention. CDC Tuberculosis. Published December 31, 2018. Accessed November 12, 2020.
- Diabetes Fact Sheets. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed November 12, 2020.
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet Gastroenterology & Hepatology. 2020;5(1):17-30
- RA Facts: What are the latest statistics on Rheumatoid Arthritis? Rheumatoid Arthritis Support Network. https://www.rheumatoidarthritis.org/ra/facts-and-statistics/. Accessed November 12, 2020.
- The Global HIV/AIDS epidemic. HIV.gov. https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics. Accessed November 12, 2020.
- Chronic Kidney Disease (CKD) Symptoms and causes. National Kidney Foundation. https://www.kidney.org/atoz/content/about-chronic-kidney-disease. Accessed November 12, 2020.
- TB comorbidities and risk factors. World Health Organization. https://www.who.int/tb/areas-of-work/treatment/risk-factors/en/. Accessed November 12, 2020.
- Lai CC, Tan CK, et al. Diagnostic performance of whole-blood interferon-γ assay and enzyme-linked immunospot assay for active tuberculosis. J Microbiol Immunol Infect. 2011 Oct;44(5):406-7. doi: 10.1016/j.jmii.2011.07.002. Epub 2011 Sep 8.
- Redelman-Sidi G, Sepkowitz KA. Interferon-gamma release assays in the diagnosis of latent tuberculosis infection among immunocompromised adults. Am J Respir Crit Care Med 2013; 188:422-431. 2012/12/25. DOI: 10.1164/rccm.201209-1621CI.
- US – Take on TB. Centers for Disease Control and Prevention. CDC Take on Tuberculosis Infographic. Accessed January 8, 2020.
- Signs & Symptoms. Centers for Disease Control and Prevention. CDC Tuberculosis Signs and Symptoms. Published March 17, 2016. Accessed January 8, 2020.
- Exposure to TB. Centers for Disease Control and Prevention. CDC Exposure to TB. Published March 21, 2016. Accessed January 8, 2020.
- TB Risk Factors. Centers for Disease Control and Prevention. CDC TB Risk Factors. Published March 18, 2016. Accessed January 8, 2020.
- Huebner R, Schein M, Bass J. The tuberculin skin test. Clin Infect Dis. 1993;968-975.
- Treatment for TB Disease. Centers for Disease Control and Prevention. CDC Treatment for TB Disease. Published April 5, 2016. Accessed January 8, 2020.