Why Diagnosing Latent Tuberculosis Infection (LTBI) in Children is Challenging
- September 23, 2021
- Evelyn Lee
- Posted in Blog
Why Diagnosing Latent Tuberculosis Infection (LTBI) in Children is Challenging

In 2019, 1.2 million children fell ill with tuberculosis (TB). 1 Healthcare providers often overlook child and adolescent TB as the disease is often difficult to diagnose due to insufficient specimen material and scarcity of bacilli in the specimen. 21
The path to diagnosing TB usually involves a few clinical investigations and tests. Some of the investigations provide hints that the body might be infected with TB, while others will require proof that the Mycobacterium TB (MTB) exists in the body especially when the patient is asymptomatic but not contagious, i.e., Latent TB infection (LTBI).
Diagnosing LTBI requires clinicians and healthcare workers to know all the investigations and test methods – how they work and what they can and cannot prove is essential, guided by WHO’s and CDC’s and local country guidelines. All together, they make up a body of evidence of TB infection.
What is Latent TB infection, and who is at risk?
Latent TB infection (LTBI) is when one is infected with the MTB but remains asymptomatic and non-contagious, which is more challenging to
diagnose than active TB infection. WHO estimates that about 25% of the world’s population has LTBI, and 5% – 15% of the LTBI population faces the
risk of progression to active TB.1 Prioritising whom to test is a critical strategy in ending TB by 2035 – one of WHO’s goals.
Within the paediatric population:
- toddlers less than 5 years of age have the highest risk of TB infection within the two years after their first infection. Those below 4 years old have the highest mortality rate. 2
- teenagers carry higher risks of reactivation to active TB after years of successful containment. 2
Children who face the highest risk are those in close contact with someone who has or had active TB or have comorbid conditions, e.g. HIV, diabetes, cardiovascular diseases, or are taking immunosuppressants with routine testing for children with HIV.
Detecting Latent Tuberculosis (LTBI)
Detecting the adaptive immune response for the T cells to the TB bacteria is essential to aid the
detection of LTBI using either the Tuberculin Skin test (TST) or the Interferon-Gamma Release
Assays (IGRAs) tests.
While the TST seems simple enough to perform and does not require phlebotomy, however, the clinical and operational limitations of the TST is well documented.3 Detection of LTBI using the TST is also a challenge to administer and interpret, especially in children; poor specificity of the test can lead to overtreatment.4
TST test and cross-reactivity with the BCG vaccine
Especially in the Asia Pacific region, where most of the population are BCG-vaccinated, the TST test
that uses the purified protein derivative (PPD) mixture cross reacts with BCG and non-tuberculous
Mycobacteria (NTM), leading to low specificity with risk of false-positive results.
TST limitations are absent in IGRAs, an ex-vivo test that detects interferon levels released by T cells on exposure to the TB antigen as an indirect measure of adaptive cell immunity. The tests use ESAT- 6 and CFP10 antigens that are more specific for MTB and not present in the BCG vaccine or NTM, limiting cross-reactivity.
Not all IGRAs are the same
Current IGRA tests use either the
ELISA
or
ELISpot Assay
technique for TB detection. While the two
techniques adopt the same principles the test design and procedures are different.
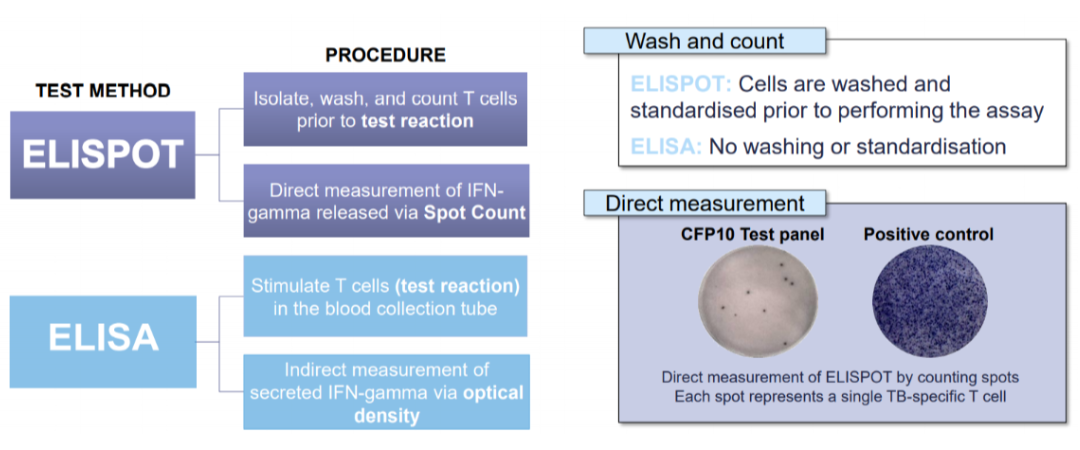
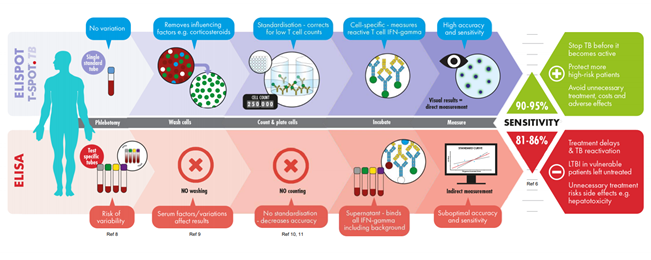
The T-SPOT®.TB test that uses the ELISPOT technique requires meticulous processing of the whole blood to isolate the peripheral blood mononuclear cells (PBMC). Cells are washed, counted, and normalised to remove variables such as granulocytes, medications, pre-circulating interferon gamma and so forth to ensure better test results even amongst the immunocompromised patients. More importantly, in a test that uses T cells to look for signs of TB infection, standardising the number of cells in each test matters: 1 million in the case of T-SPOT.TB
Test accuracy matters
The Mandalakas et al. (2018) study evaluated the performance of T-SPOT.TB in a low TB burden setting using 6.54 million samples.
The results were conclusive and correlated well in 98% of cases, doing away with the uncertainties of intermediate results.5 In the Rego et al (2018)study of
645,947 amples the T-SPOT.TB had an invalid rate of less than 1%.6
In a Hong Kong study (2015) the T-SPOT.TB outperformed TST in a BCG-vaccinated population of 99%. Using a TST cut-off at 15mm, the standard cut-off in BCG vaccinated patients, 56% of latent TB and 62.5% of active TB disease patients were missed. This study highlights the importance of using T-SPOT.TB in areas with high BCG vaccination and low risk of ongoing transmission.7
A study by Lamb et al (2020) involving 5,990 children from high-burden TB countries seeking refuge in the US demonstrated that T-SPOT.TB had fewer false-positive results than TST. The results also suggest that effects of BCG-vaccination on TST may be more prolonged than expected. The CDC has now updated its pre-immigration screening guidelines and recommends using IGRA rather than TST in children from high-TB incidence countries.8
It is also essential to understand the differences in design and interpretation of the two IGRA tests. While the vast majority of T-SPOT.TB tests are either positive or negative, a small percentage can fall under the borderline or indeterminate categories
The borderline category – not found in ELISA tests, is designed to reduce the likelihood of false-positive or false-negative results around the cut-off point of the T-SPOT.TB test. As opposed to an indeterminate or invalid result, a borderline result is clinically interpretable. It should be followed by retesting as a substantial proportion of individuals may test positive upon retesting. Almost 80% of patients testing borderline are resolved as positive or negative on retesting, which helps maintain the test’s high sensitivity and specificity and reduced uncertainties in diagnosis.6
Guidelines and clinical judgement shape how we test
Amongst the high-risk paediatric population, those who have comorbid conditions but without exposure to TB: Do they need to be tested and treated for LTBI?
In TB, 90% of the time, history and the physical exam shape the clinical judgement with tests such as IGRAs, TST, chest X-rays, cultures to help drive the treatment decision.
Taking the child’s historical context becomes a critical determinant and should shape the clinician’s decision on the type of test to use as the first step towards detecting LTBI. For example, if the child:
- has had the BCG vaccine: The IGRA test should be used instead of the TST.
- been validated in diabetes, rheumatological, gastrointestinal, dermatological and renal diseases, and in patients with HIV and immunosuppressive conditions.9 It has proven accuracy in ruling out TB in HIV and immunocompromised patients with a negative predictive value of 100% in this population.10
- is less than 5 years old: Clinical guidelines from WHO, MOH Singapore, Korea, AAP, IDSA, ATS and CDC, recommend using IGRA over TST in children above 5 years. However, clinicians could consider using T-SPOT.TB as it is the only test with no FDA limitation for use in children over 2 years of age.
Contact tracing needs to be part of the investigation process. Coupled with screening, clinicians can help detect cases actively before the child is sick, which is difficult if the child does not display typical TB symptoms.
Diagnosing TB is a perpetual challenge, especially with toddlers. It requires choosing the right test used along with other clinical data to support the diagnosis. Testing becomes the first step to end TB mortality amongst the paediatric population.
References
- World Health Organization: Tuberculosis: Key Facts. (2020 October 14). https://www.who.int/news-room/fact-sheets/detail/tuberculosis
- Thomas, T.A. (2017, August). Tuberculosis in Children. Pediatr Clin N Am. 64(4), 893 – 909.
- Gualano, G., Mencarini, P., Lauria, F. N., Palmieri, F., Mfinanga, S., Mwaba, P., Jeremiah, C., Zumla, A., Ippolito, G. (2019). Tuberculin skin test–Outdated or still useful for Latent TB infection screening?. Int J Infect Dis. 80, S20-S22.
- MaWhitehall, J. (2015, June 2) Poor specificity of TB skin test may lead to overtreatment in children. Healio.com. https://www.healio.com/news/pediatrics/20150602/poor-specificity-of-tb-skin-test-may-lead-to-overtreatment-in-children
- Mandalakas, A. M., Highsmith, H. Y., Harris, N. M., Pawlicka, A., & Kirchner, H. L. (2018). T-SPOT. TB Performance in routine pediatric practice in a low TB burden setting. The Pediatric Infectious Disease Journal, 37(4), 292-297.
- Rego, K., Pereira, K., MacDougall, J., & Cruikshank, W. (2018). Utility of the T-SPOT®. TB test’s borderline category to increase test resolution for results around the cut-off point. Tuberculosis. 2018;108, 178-185
- Leung, C. C., Yam, W. C., Ho, P. L., Yew, W. W., Chan, C. K., Law, W. S., … & Tam, C. M. (2015). T‐S pot. TB outperforms tuberculin skin test in predicting development of active tuberculosis among household contacts. Respirology, 20(3), 496-503.
- Lamb, G. S., Cruz, A. T., Camp, E. A., Javier, M., Montour, J., Piper, T., Shah, U.A, Starke, J. R. (2020). Tuberculosis in Internationally Displaced Children Resettling in Harris County, Texas, USA, 2010–2015. Emerg Infect Dis. 26(8), 2020./.
- Oxford Immunotec. T-SPOT.TB Package Insert PI-TB-US-0001 V9. Abingdon, UK. February 2021
- Sun, H. Y., Hsueh, P. R., Liu, W. C., Su, Y. C., Chang, S. Y., Hung, C. C., & Chang, S. C. (2015). Risk of active tuberculosis in HIV-infected patients in Taiwan with free access to HIV care and a positive T-SPOT. TB test. PloS one, 10(5), e0125260.