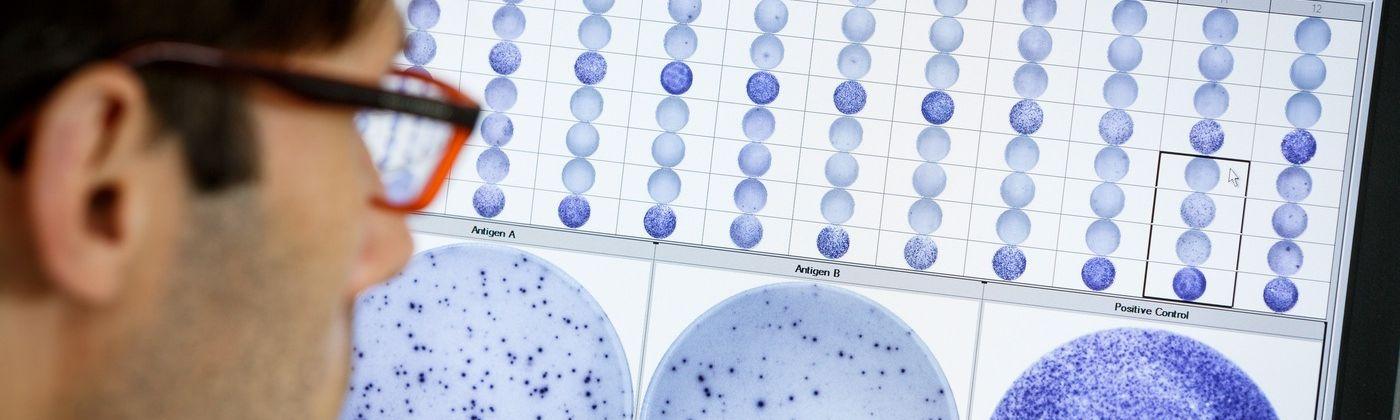
Performance
The T-SPOT.TB test difference
For many years, there was only one way to test for tuberculosis (TB): the tuberculin skin test (TST). This is no longer the case, as there are now two categories of tests to detect TB infection:

The tuberculin skin test1
The TST has been used to detect TB infection for over 100 years. They require an intradermal injection of a small amount of purified protein derivative (PPD), a TB antigen, into the skin. In 48-72 hours, the resultant induration is measured.
Interferon-gamma release assays
Interferon-gamma release assays (IGRAs), which include the T-SPOT®.TB test, are blood-based tests. The technology is based on the release of interferon-gamma secreted by individual effector T cells (both CD4+ and CD8+) after being stimulated by TB-specific antigens.
Accurate across patient populations |
||||
| TB test considerations | IGRAs | TST | ||
|---|---|---|---|---|
| T-SPOT®.TB2 | QFT®-Plus3 | LIAISON® QFT-Plus4 | ||
| Utility in immunocompromised patients |
|
|
Studies have |
|
|
Sensitivity and specificity > 95% |
||||
| FDA-approved borderline zone | ||||
|
No cross-reactivity with BCG vaccine |
||||
|
Objective test results |
||||
Consistent results |
||||
| TB test considerations | T-SPOT®.TB | QFT®-Plus | LIAISON® QFT-Plus4 | TST |
|---|---|---|---|---|
| < 1% uninterpretable test results | N/A | |||
| Converison rate < 1% in serial testing | QFT®-Gold 4.4%8 |
N/A* | N/A | |
One tube with no refrigeration |
||||
| TB test considerations | T-SPOT®.TB2 | QFT®-Plus3 | LIAISON® QFT-Plus4 | TST |
|---|---|---|---|---|
| On-site incubation or refrigeration of specimen is never required | N/A | |||
| Single, streamlined blood collection and process |
|
|
N/A | |
| A single visit is required |
|
|||
Unique CPT® code9 |
|
||
| CPT® code | 86481* | 86480* | 86480* |
|---|---|---|---|
| Applicable test | The T-SPOT.TB test | QuantiFERON®-TB Gold Plus (QFT®-Plus) | LIAISON® QFT-Plus |
| Description | Tuberculosis test, cell mediated immunity antigen response measurement; enumeration of gamma interferon-producing T-cells in cell suspension | Tuberculosis test, cell mediated immunity measurement of gamma interferon producing antigen response | Tuberculosis test, cell mediated immunity measurement of gamma interferon producing antigen response |